what test is used to measure the concentration of urine?
| Urine test strip | |
|---|---|
 The Multistix urine examination strip showing the manufacturer's coloured scale. | |
| Purpose | determine pathological changes |
A urine exam strip or dipstick is a bones diagnostic tool used to determine pathological changes in a patient'south urine in standard urinalysis.[ane]
| Leukocytes | |
| Nitrite | |
| Urobilinogen | |
| Protein | |
| pH | |
| Haemoglobin | |
| Specific gravity | |
| Ketone | |
| Bilirubin | |
| Glucose | |
A standard urine test strip may comprise up to 10 different chemical pads or reagents which react (change color) when immersed in, and then removed from, a urine sample. The examination tin often be read in as fiddling as threescore to 120 seconds after dipping, although certain tests require longer. Routine testing of the urine with multiparameter strips is the beginning pace in the diagnosis of a wide range of diseases. The analysis includes testing for the presence of proteins, glucose, ketones, haemoglobin, bilirubin, urobilinogen, acetone, nitrite and leucocytes as well as testing of pH and specific gravity or to test for infection past different pathogens.[ii]
The test strips consist of a ribbon made of plastic or paper of almost 5 millimetre wide, plastic strips have pads impregnated with chemicals that react with the compounds present in urine producing a characteristic color. For the newspaper strips the reactants are absorbed directly onto the paper. Newspaper strips are often specific to a single reaction (eastward.one thousand. pH measurement), while the strips with pads allow several determinations simultaneously.[ii]
In that location are strips which serve different purposes, such every bit qualitative strips that only determine if the sample is positive or negative, or there are semi-quantitative ones that in add-on to providing a positive or negative reaction also provide an interpretation of a quantitative result, in the latter the colour reactions are approximately proportional to the concentration of the substance being tested for in the sample.[2] The reading of the results is carried out by comparison the pad colours with a colour calibration provided by the manufacturer, no additional equipment is needed.[3]
This blazon of analysis is very mutual in the control and monitoring of diabetic patients.[2] The fourth dimension taken for the appearance of the exam results on the strip can vary from a few minutes after the examination to 30 minutes afterwards immersion of the strip in the urine (depending on the make of product beingness used).
Semi-quantitative values are usually reported equally: trace, one+, 2+, 3+ and 4+; although tests tin also be estimated equally milligrams per decilitre. Automated readers of examination strips also provide results using units from the International Organization of Units.[ii]
Test method [edit]
The examination method consists of immersing the test strip completely in a well mixed sample of urine for a short period of time, so extracting it from the container and supporting the border of the strip over the mouth of the container to remove backlog urine. The strip is then left to stand for the time necessary for the reactions to occur (usually ane to 2 minutes), and finally the colours that appear are compared against the chromatic calibration provided past the manufacturer.
An improper technique tin can produce simulated results, for example, leukocytes and erythrocytes precipitate at the bottom of the container and may not exist detected if the sample is not properly mixed, and in the same style, if an excess of urine remains on the strip subsequently information technology has been removed from the test sample, may cause the reagents to leak from the pads onto adjacent pads resulting in mixing and distortion of the colours. To ensure that this does not occur information technology is recommended the edges of the strip are dried on absorbent paper.[2]
Reactions for generalised tests [edit]

Comparing between ii reactive strips, one pathological (to the left, from a patient with uncontrolled diabetes mellitus), and an unreacted strip. From tiptop to bottom the pathological strip shows: Leukocytes (-), nitrites (-), urobilinogen (-), proteins (+), pH (5), hemoglobin (+), specific gravity (ane.025), ketones (++++), bilirubin (+), glucose (+++).
pH [edit]
The lungs and kidneys are the main regulators of an organism'southward acid / alkali balance. The balance is maintained through the controlled excretion of acidic hydrogens in the form of ammonia ions, monohydrogenated phosphate, weak organic acids and through the reabsorption of bicarbonate through glomerular filtration in the convoluted tubules of the nephron. The pH of urine normally vary between 4.5 and viii with the get-go urine produced in the morning generally being more acidic and the urine produced later on meals mostly more alkaline.[4] Normal reference values are not provided for urine pH as the variation is too wide and results take to be considered in the context of the other quantifiable parameters.[4]
The determination of urinary pH has two principal objectives, 1 is diagnostic and the other is therapeutic. On the one hand it provides information regarding the balance between acid and alkali in a patient and allows identification of the substances that are present in the urine in crystalline grade. On the other mitt, certain illnesses crave a patient to proceed the pH of their urine within given narrow margins, whether to promote the elimination of chemotherapeutic agents, avert the precipitation of salts that promote the formation of kidney stones, or in gild to facilitate the control of a urinary infection. Regulating nutrition mainly controls urinary pH, although using medication tin besides control information technology. Diets rich in creature proteins tend to produce acidic urine, while diets mainly composed of vegetables tend to produce alkali urine.[4]
Commercial brands mensurate pH in increments of 0.5 or 1 pH units between pH 5 and nine. In guild to differentiate pH in this wide range it is mutual to use a double indicator system comprising methyl red and bromothymol blue.[v] Methyl crimson produces a colour change from ruby to yellow in the range of pH 4 to 6 and the bromothymol bluish changes from yellow to blue between pH vi and ix. In the range 5 to 9 the strips show colours that change from orange at pH 5, passing through xanthous and greenish to night blue at pH ix.[vi]
Specific gravity [edit]
One of the kidneys' important functions is to reabsorb water after glomerular filtration. The complex procedure of reabsorption is normally one of the first renal functions to be affected past disease. The specific gravity of urine is a measure of its density compared to H2O and depends on the quantity and density of solutes (molecules with more than mass per volume increase mensurate of specific gravity). The measurement of specific gravity should non be confused with the measurement of osmotic concentration, which is more related to the number of particles than with their mass.[vii]
The urine test strip test for specific gravity is based on the change in dissociation abiding (pKa) of an anionic polyelectrolyte (poly-(methyl vinyl ether/maleic anhydride)) in an alkali medium that is ionised and releases hydrogen ions in proportion to the number of cations present in the solution.[six] The greater the cation concentration of the urine the more hydrogen ions are released, thereby reducing the pH. The pad also includes bromothymol bluish, which measures this change in pH.[6] [8] It should be remembered that the test strip only measures cation concentration, information technology is therefore possible that urine with a loftier concentration of non-ionic solutes (such as glucose or urea) or with high molecular weight compounds (such as the media used to provide radiographic contrast) volition yield a result that will exist erroneously lower than that measured past densitometry. The colours vary from night blueish with a reading of one.000 to yellowish for a reading of 1.030.[eight] [ix]
- In an alkaline medium
Polyelectrolyte-Hn + Cationsn+ → Polyelectrolyte-Cations + nH+
- In an alkaline medium
H+ + Bromothymol blue(Blue) → Bromothymol blue-H+ (Yellow)
Elevated protein concentrations produce slightly elevated specific density results as a upshot of the indicator'due south protein error; in add-on, samples with a pH in a higher place 6.5 give lower readings as a result of the indicator's bias. For this reason the manufacturers recommend that 5 units are added to the specific gravity reading when the pH is greater than 6.v.[8]
Blood [edit]
Blood may be present in the urine either in the form of intact red blood cells (hematuria) or as the product of cerise claret cell destruction, hemoglobin (hemoglobinuria). Blood present in large quantities can be detected visually. Hematuria produces cloudy ruby urine, and hemoglobinuria appears as a clear carmine specimen. Any corporeality of blood greater than five cells per microliter of urine is considered clinically significant; visual exam cannot exist relied upon to detect the presence of blood. Microscopic examination of the urinary sediment shows intact red claret cells, just free hemoglobin produced either by hemolytic disorders or lysis of red blood cells is not detected. Therefore, chemical tests for hemoglobin provide the most accurate hateful for determining the presence of blood. Once blood has been detected, the microscopic examination can exist used to differentiate between hematuria and hemoglobinuria.
Chemical tests for blood apply the pseudoperoxidase action of hemoglobin to catalyze a reaction betwixt the heme component of both hemoglobin and myoglobin and the chromogen (a substance that acquires colour after a chemical reaction) tetramethylbenzidine to produce an oxidized chromogen, which has a greenish-blue colour. Reagent strip manufacturers comprise peroxide, and tetramethylbenzidine, into the blood testing area. Two colour charts are provided that correspond to the reactions that occur with hemoglobinuria, myoglobinuria and hematuria (RBCs). In the presence of free hemoglobin/myoglobin, compatible color ranging from a negative yellow through green to a strongly positive green-blue appears on the pad. In contrast, intact ruby-red blood cells are lysed when they come up in contact with the pad, and the liberated hemoglobin produces an isolated reaction that results in a speckled pattern on the pad. Reagent strip tests tin can discover concentrations every bit low equally five red blood cells per microliter; withal, care must exist taken when comparing these figures with the bodily microscopic values, because the absorbent nature of the pad attracts some of urine. The terms trace, small, moderate, and large (or trace, 1+, two+, and 3+) are used for reporting.
Imitation-positive reactions due to menstrual contamination may be seen. They also occur if stiff oxidizing detergents are nowadays in the specimen container. Vegetable peroxidase and bacterial enzymes, including an Escherichia coli peroxidase, may also cause false-positive reactions. Therefore, sediments containing bacteria should be checked closely for the presence of crimson claret cells. Traditionally, ascorbic acid (vitamin C) has been associated with false-negative reagent strip reactions for blood. Both Multistix and Chemstrip have modified their reagent strips to reduce this interference to very high levels of ascorbic acid, and Chemstrip overlays the reagent pad with an iodate-impregnated mesh that oxidizes the ascorbic acid prior to its reaching the reaction pad. False-negative reactions tin can consequence when urine with a high specific gravity contains crenated red blood cells that do non lyse when they come in contact with the reagent pad. Decreased reactivity may also be seen when formalin is used as a preservative or when the hypertension medication captopril or high concentration of nitrite are present. Red blood cells settle to the lesser of the specimen container, and failure to mix the specimen prior to testing causes a falsely decreased reading.[ten]
Diseases identified [edit]
With the aid of routine examinations early on symptoms of the following 4 groups can exist identified:
- Diseases of the kidneys and the urinary tract
- Carbohydrate metabolism disorders (diabetes mellitus)
- Liver diseases and haemolytic disorders
- Urinary infections
Urinary tract [edit]
Screening parameters: Many renal and urinary tract diseases may be asymptomatic for a long period of time. Routine urinalysis is recommended every bit a basic notwithstanding fundamental stride in identifying renal impairment and/or urinary tract disease at an early phase, particularly in high-risk populations such every bit diabetics, the hypertensive, African Americans, Polynesians, and those with a family history.[11]
Specific kidney and urinary tract diseases that tin be identified include: chronic kidney disease, glomerulonephritis, proteinuria and haematuria.
Poly peptide testing [edit]
Of the routine chemical tests performed on urine, the most indicative of renal disease is the protein determination. Proteinuria is often associated with early renal disease, making the urinary poly peptide test an important role of whatever physical examination. Normal urine contains very little poly peptide, usually less than 100–300 mg/Fifty or 100 mg per 24 hours is excreted. This protein consists primarily of low-molecular-weight serum proteins that have been filtered past the glomerulus and proteins produced in the genitourinary tract. Due to its low molecular weight, albumin is the major serum poly peptide found in the plasma, the normal urinary albumin content is low because the majority of albumin presented in the glomerulus is not filtered, and much of the filtered albumin is reabsorbed by the tubules. Other proteins include pocket-size amounts of serum and tubular microglobulins. Uromodulin produced by the renal tubular epithelial cells and proteins from prostatic, seminal, and vaginal secretions. Uromodulin is routinely produced in the distal convoluted tube, and forms the matrix of casts.
Traditional reagent strip testing for protein uses the principle of the protein error of indicators to produce a visible colorimetric reaction. Contrary to the general conventionalities that indicators produce specific colours in response to detail pH levels, certain indicators change colour in the presence of protein even though the pH of the medium remains constant. This is so considering poly peptide accepts hydrogen ions from the indicator. The examination is more sensitive to albumin because albumin contains more amino groups to accept the hydrogen ions than other proteins. Depending on the manufacturer, the protein area of the strip contains dissimilar chemicals. Multistix contains tetrabromophenol blue and Chemstrip contains three',3",five',5"-tetrachlorophenol, 3,4,5,6-tetrabromosulfonphthalein. Both contain an acrid buffer to maintain the pH at a constant level. At a pH level of 3, both indicators announced yellow in the absence of protein. However, equally the poly peptide concentration increases, the color progresses through various shades of green and finally to blue. Readings are reported in terms of negative, trace, 1+, ii+, 3+ and 4+ or the semi-quantitative values of 30, 100, 300 or 2000 mg/dL respective to each color change. Trace values are considered to be less than xxx mg/dL. Estimation of trace readings can exist difficult.[12]
Indicator-H+ (Yellow) + Protein → Indicator(Blue-greenish) + Poly peptide-H+
The major source of fault with reagent strips occurs with highly buffered element of group i urine that overrides the acid buffer organisation, producing a rise in pH and a colour change unrelated to protein concentration. Likewise, a technical error of allowing the reagent pad to remain in contact with the urine for a prolonged flow may remove the buffer. False-positive readings are obtained when the reaction does not have place under acidic conditions. Highly pigmented urine and contamination of the container with fourth ammonium compounds, detergents and antiseptics also cause imitation-positive readings. A false-positive trace reading may occur in specimens with a high specific gravity.
Hemoglobin and myoglobin testing [edit]
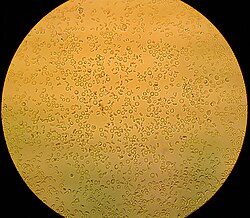
Microphotograph of a macroscopic hematuria, the biconcave form of the red claret cells is clearly visible, it is rare to find examples in such a well conserved condition.
The presence of claret in the urine is, of all the parameters unremarkably tested, the one that is most closely related with traumatic damage to the kidneys or the genitourinary tract. The nearly common causes of hematuria are: nephrolithiasis, glomerular affliction, tumours, pyelonephritis, exposure to nephrotoxins, and handling with anticoagulants. Non-pathological hematuria can exist observed after strenuous exercise and during menstruation. The normal number of red blood cells in urine should not usually exceed 3 per high power field.[13]
A urine test strip showing positive for blood can likewise indicate hemoglobinuria, which is not detectable using a microscope due to the lysis of red claret cells in the urinary tract (especially in element of group i or dilute urine), or intravascular hemolysis. Under normal weather the formation of haptoglobin-hemoglobin complexes prevents glomerular filtration, simply if the hemolysis is extensive haptoglobin's uptake capacity is exceeded and hemoglobin can appear in urine. Hemoglobinuria can be caused by hemolytic anaemia, blood transfusions, all-encompassing burns, the bite of the recluse spider (Loxosceles), infections and strenuous exercise.
The urine exam strip examination for blood is based on hemoglobin'southward pseudo peroxidase activity in catalysing a reaction between hydrogen peroxide and the chromogen tetramethylbenzidine in order to produce a dark blue oxidation product.[half-dozen] [13] the resultant colour can vary between dark-green and nighttime blue depending on the amount of hemoglobin.[13]
- Catalysed by hemoglobin acting as a peroxidase
HtwoOtwo + Chromogen → Oxidised chromogen (coloured) + H2O
The reaction is not only catalysed past blood hemoglobin, other globins with a hem group such as myoglobin can also catalyse the same reaction.[13]
The presence of myoglobin in urine gives a positive reaction in the test strip'south blood test but the urine appears clear with a crimson to brown colouration. The presence of myoglobin in place of hemoglobin can be caused by pathologies associated with muscular damage (rhabdomyolysis), such every bit trauma, crush syndrome, prolonged coma, convulsions, progressive muscular atrophy, alcoholism, heroin abuse and strenuous physical activity.
The haem fraction of these proteins is toxic for the kidney tubules and elevated concentrations tin crusade acute kidney injury.
It is possible to use an ammonia sulphate precipitation test in order to distinguish between hemoglobinuria and myoglobinuria. This consists of adding 2.8gr of ammonia sulphate to five ml of centrifuged urine, mixing well and afterwards five minutes filtering the sample and centrifuging again. The hemoglobin precipitates out with the ammonia sulphate merely not the myoglobin. Analysis of the supernatant for blood with a test strip will requite a positive if myoglobin is nowadays and a negative if hemoglobin is present.
The exam can give false positives if strong oxidant or peroxide residues are present on the laboratory cloth used for the assay.[13]
Carbohydrate disorders [edit]
- Glucose - Identified as Glycosuria
- Ketones - Identified as Ketonuria (besides see ketoacidosis and ketosis)
Effectually 30–40% of type I diabetics and around xx% of type II diabetics suffer in time from a nephropathy, and early recognition of diabetes is therefore of major significance for the farther state of health of these patients.
Specific sugar metabolism disorders able to be identified include Diabetes Mellitus, Glucosuria and Ketonuria.
Glucose test [edit]
Under normal conditions almost all the glucose removed in the glomerulus is reabsorbed in the proximal convoluted tubule. If the blood glucose level increases, equally happens in diabetes mellitus, the capacity of the convoluted tubule to reabsorb glucose is exceeded (an result known equally renal reabsorption threshold). For glucose this threshold is between 160–180 mg/dl. Glucose concentrations vary in an individual, and a healthy person can nowadays with transitory glucosuria subsequently a repast high in sugars; therefore the most representative results come up from samples obtained at least two hours afterwards food is eaten.
The detection of glucose past test strips is based on the enzymatic reaction of glucose oxidase. This enzyme catalyses the oxidation of glucose by atmospheric oxygen to form gluconic acid and hydrogen peroxide. A second linked reaction, mediated by a peroxidase, catalyses the reaction between the peroxide and a chromogen (a substance that acquires colour afterwards a chemical reaction) to form a coloured compound that indicates the glucose concentration.[vi]
-
- 1) Catalysed by glucose oxidase
Glucose + Oii → D-glucono-δ-lactone + H2O2
- 1) Catalysed by glucose oxidase
-
- two) Catalysed by peroxidase
HiiO2 + Chromogen → Oxidised chromogen (coloured) + H2O
- two) Catalysed by peroxidase
The reaction is specific for glucose, as occurs in all enzymatic reactions, only it tin provide some false positive results due to the presence of traces of strong oxidising agents or peroxide from disinfectants used on laboratory instruments.[6]
Ketone test [edit]
The term ketones or ketone bodies in reality refers to three intermediate products in the metabolism of fatty acids; acetone, acetoacetic acid and beta-hydroxybutyric acid. Elevated concentrations of ketones are not generally found in urine, equally all these substances are completely metabolized, producing energy, carbon dioxide and water. Still, the disruption of carbohydrate metabolism can lead to metabolic imbalances and the appearance of ketones as a by-product of the metabolism of an organism'south fat reserves.
An increase in fat metabolism tin exist the result of starvation or malabsorption, the disability to metabolize carbohydrates (every bit occurs, for instance, in diabetes) or due to losses from frequent airsickness.
The command of urinary ketone is particularly useful in managing and monitoring diabetes mellitus type 1. Ketonuria indicates an insulin deficiency that indicates the need to regulate its dosage. An increase in the blood concentration of ketone produces a water-electrolyte imbalance, dehydration and if not corrected, acidosis and in the end diabetic coma.
The three ketone compounds appear in dissimilar proportions in the urine, although these proportions are relatively constant in different samples as both the acetone and the beta-hydroxybutyric acid are produced from the acetoacetic acid. The proportions are 78% beta-hydroxybutyric acrid, 20% acetoacetic acid and 2% acetone.
The test used in the urine exam strips is based on the reaction of sodium nitroprusside (nitroferricyanide). In this reaction the acetoacetic acid in an alkali medium reacts with the sodium nitroprusside producing a magenta coloured complex:[half-dozen] [14]
-
Natwo[Atomic number 26(CN)5NO] + CHthreeCOCH2COOH + 2Na(OH) → Naiv[Atomic number 26(CN)five-N=CHCOCH2COOH](magenta) + H2O
-
Sodium nitroprusside + Acetoacetic acid + Brine medium → Pink-magenta complex + Water
The examination does non measure beta-hydroxybutyric acid and it is only weakly sensitive to acetone when glycine is added to the reaction. Yet, as these compounds are derived from the acetoacetic acrid their existence can be assumed and a separate test is not therefore necessary. Those medicines that contain sulfhydryl groups, such as mercaptoethane sulphonate Na (Mesna) and captopril and Fifty-DOPA can give atypical colouring. A false negative can occur in samples that have non been adequately stored due to volatilization and bacterial degradation.
Liver and blood disorders [edit]
In many liver diseases the patients frequently show signs of pathology only at a late stage. Early on diagnosis allows advisable therapeutic measures to be instituted in good time, avoiding consequential harm and further infections.
Specific liver diseases and haemolytic disorders able to exist identified include liver illness, (accompanied by jaundice), cirrhosis, urobilinogenuria and bilirubinuria.
Bilirubin test [edit]
Bilirubin is a highly pigmented compound that is a past-product of haemoglobin degradation. The haemoglobin that is released after the mononuclear phagocyte system (located in the liver and spleen) withdraws former red blood cells from apportionment is degraded into its components; fe, protoporphyrin and protein. The system's cells catechumen the protoporphyrin into unconjugated bilirubin that passes through the circulatory organization leap to protein, specially albumin. The kidney is unable to filter out this bilirubin every bit it is bound to poly peptide, however, it is conjugated with glucuronic acid in the liver to form water-soluble conjugated bilirubin. This conjugated bilirubin does non ordinarily appear in the urine equally information technology is excreted directly from the intestine in bile. Intestinal bacteria reduce the bilirubin to urobilinogen, which is afterward oxidised and either excreted with the faeces as stercobilin or in the urine as urobilin.
Conjugated bilirubin appears in urine when the normal deposition cycle is altered due to the obstruction of the biliary ducts or when the kidney'due south functional integrity is damaged. This allows the escape of conjugated bilirubin into the circulation as occurs in hepatitis and hepatic cirrhosis).
The detection of urinary bilirubin is an early indication of liver disease and its presence or absence can be used to make up one's mind the causes of clinical jaundice.
The jaundice produced past the accelerated devastation of crimson claret cells does not produce bilirubinuria, as the high serum bilirubin is found in the unconjugated grade and the kidneys are unable to excrete it.
The examination strips use a diazotization reaction in order to detect bilirubin. The bilirubin combines with a diazonium salt (2,4-dichloroaniline or two,half-dozen-dichlorobenzene-diazonium-tetrafluoroborate) in an acrid medium to produce an azo dye with colouration that varies from pinkish to violet:[6]
-
- In acrid medium
Bilirubin glucuronide + Diazonium salt→ Azo dye (violet)
- In acrid medium
Fake positive reactions can be due to unusual pigments in the urine (for case, yellowy orange phenazopyridine metabolites, indican and the metabolites of the medicine Lodine (Etodolac)). Faux negatives tin can as well be given by poorly stored samples as the bilirubin is photosensitive and undergoes photo oxidation to biliverdin when it is exposed to light, or hydrolysis of the glucuronide tin occur producing free bilirubin which is less reactive.[6]
Urobilinogen test [edit]
Abdominal bacteria convert the conjugated bilirubin that is excreted by the bile duct into the intestine into urobilinogen and stercobilinogen. Part of the urobilinogen is reabsorbed in the intestine and so circulated in the blood to the liver where it is excreted. A small-scale role of this recirculated urobilinogen is filtered out past the kidneys and appears in urine (less than 1 mg/dl urine). The stercobilinogen can not be reabsorbed and remains in the intestine.[15] [16]
Any deterioration in liver function reduces its ability to process the recirculated urobilinogen.[fifteen] The excess that remains in the claret is filtered out past the kidneys and appears in urine. When hemolytic disorders occur the amount of unconjugated bilirubin that is present in the claret increases causing an increase in hepatic excretion of conjugated bilirubin, resulting in increased amounts of urobilinogen that in turn causes an increase in reabsorption, recirculation and renal excretion.[fifteen] [16]
The reactions that take place in the test strip vary co-ordinate to the manufacturer, simply in reality there are two reactions that are most frequently used. Some manufacturers use Ehrlich'southward reaction (1), in which urobilinogen reacts with p-dimethylaminobenzaldehyde (Ehrlich's reagent) in order to produce colours that vary from light to dark pink. Other manufacturers use a diazo coupling reaction (2) that uses iv-methoxybenzene-diazonium-tetrafluoroborate to produce colours that vary from white to pink. The latter reaction is more specific.[17]
-
- (i) Reaction on Multistix (in acid medium)
Urobilinogen + p-dimethylaminobenzaldehide → Red dye
- (i) Reaction on Multistix (in acid medium)
-
- (ii) Reaction on Chemstrip (in acrid medium)
Urobilinogen + 4-methoxibenzene-diazonium-tetrafluoroborate → Red azo dye
- (ii) Reaction on Chemstrip (in acrid medium)
A number of substances interfere with the Ehrlich reaction on the Multistix strip: porphobilinogen, indican, p-amino salicylic acrid, sulphonamide, methyldopa, procaine and chlorpromazine. The exam should be carried out at room temperature as the reaction's sensitivity increases with temperature. Poorly stored samples can yield false negative results equally the urobilinogen suffers photo oxidation to urobilin that does not react. The formaldehyde used as a preservative produces simulated negatives in both reactions.[16]
Urinary infections [edit]
Urinary infections tin can be identified including bacteriuria and pyuria.
Nitrites test [edit]
The examination for nitrites is a rapid screening method for possible asymptomatic infections caused past nitrate-reducing leaner. Some of the gram negative leaner species that about commonly cause urinary tract infections (Escherichia coli, Enterobacter, Klebsiella, Citrobacter and Proteus) have enzymes that reduce the nitrate present in urine to nitrite.[xviii] The test is a rapid screen for possible infections by enteric bacteria, simply it does not replace the urinalysis tests nor microscopic examination as diagnostic tools, nor subsequent monitoring as many other microorganisms that do not reduce nitrate (gram positive leaner and yeasts) tin can as well cause urinary infections.[nineteen] [20]
The reactive strips detect nitrite past using the Griess reaction in which the nitrite reacts in an acrid medium with an aromatic amine (para-arsanilic acrid or sulphanilamide) in social club to form a diazonium salt that in plow reacts with tetrahydrobenzoquinoline to produce a pink azo dye.[6] [20]
-
- 1) In an acid medium
Para-arsanilic acid or sulphanilamide + NO −
2 → Diazonium salt
- 1) In an acid medium
-
- 2) In an acid medium
Diazonium common salt + tetrahydrobenzoquinoline → Pink azo dye
- 2) In an acid medium
The nitrite examination is non particularly reliable and negative results in the presence of clinical symptoms are not uncommon, meaning that the test should non exist taken as conclusive. Negative results tin be obtained in the presence of non nitrate-reducing microorganisms. Nitrite-reducing bacteria need to remain in contact with nitrate for long enough to produce detectable amounts (first urine produced in the morning or at least with a urine retentivity of 4 hours). Large numbers of bacteria tin can react to reduce nitrite to nitrogen, which will requite a simulated negative effect. The utilize of antibiotics volition inhibit bacterial metabolism causing negative results even though bacteria are present. In add-on some substances such every bit ascorbic acid will compete with the Greiss reaction giving unrepresentatively low readings.[half dozen] [xx]
Leukocytes exam [edit]

A sample of urine sediment from a patient suffering from a urinary infection, it is possible to see leukocytes (small-scale round and granular), erythrocytes (small-scale round and biconcave) and epithelial cells (large and polyhedral). The test for leukocyte esterase is indicative and does not supercede microscopic exam of urine.[nineteen]
It is normal to detect up to 3 (occasionally 5) leukocytes per loftier power field (40X) in a urine sample, with women having slightly higher results owing to vaginal contamination.[ citation needed ] Higher numbers indicate urinary infection. The urine test strip exam for white blood cells detects leukocyte esterase, which is nowadays in azurophilic granules of monocytes and granulocytes (neutrophilic, eosinophilic and basophilic). Bacteria, lymphocytes and epithelial cells from the genitourinary tract practise not contain esterases.[21] Neutrophil granulocytes are the leukocytes nigh commonly associated with urinary infections. A positive examination for leukocyte esterase usually indicates the presence of bacteria and a positive nitrite test (although information technology is not always the instance). Infections caused past Trichomonas, Chlamydia and yeasts produce leukocyturia without bacteriuria. The inflammation of the renal tissues (interstitial nephritis) can produce leukocyturia, in particular toxic interstitial nephritis with predominant eosinophils.[21]
The exam for leukocyte esterase is purely indicative and should not exist solely relied on for diagnosis, every bit it does not supplant microscopic or urine culture examinations.[19]
The urine examination strip reaction is based on the action of leukocyte esterase in catalysing the hydrolysis of an ester of indolecarboxylic acrid. The indoxyl that is liberated combines with a diazonium salt in club to produce a violet coloured azole dye.[21]
-
- 1) Reaction catalysed by leukocyte esterase
Indolecarboxylic acid ester → Indoxyl + Acrid
- 1) Reaction catalysed by leukocyte esterase
-
- 2) In acid medium
Indoxyl + Diazonium salt → Violet azole dye
- 2) In acid medium
The esterase reaction needs about 2 minutes to accept identify. The presence of strong oxidising agents or formaldehyde can cause false positives. False negative results are associated with elevated concentrations of protein (greater than 500 mg/dL), glucose (greater than three 1000/dL), oxalic acid and ascorbic acid. Urine with a high specific gravity tin can likewise crusade leukocyte crenation, which tin can impede the liberation of the esterases.[22]
Detection limit [edit]
The detection limit of a test is the concentration at which the test starts to turn from negative to positive. Although the detection limit may vary between urine samples, the detection limit is defined as the concentration of the analyte that results in a positive reaction in 90% of the examined urines.
| Parameter | Reference range | Practical detection limit |
|---|---|---|
| Specific Gravity Reference range Physiological range | 1.016–1.022 i.002–1.035 | Range: 1.000–1.030 |
| pH value Commencement morning urine During the day | 5–vi iv.8–7.iv | Range: 5–9 |
| Leukocytes Reference range Gray zone | < 10 Leu/µl x–20 Leu/µl | ten–25 Leu/µl |
| Nitrite | – | 0.05 mg/dl (xi µmol/l) |
| Poly peptide Albumin | < two mg/dl | half-dozen mg/dl |
| Glucose First morning urine During the mean solar day | < xx mg/dl < 30 mg/dl | forty mg/dl (2.two mmol/fifty) |
| Ketones Acetoacetic acrid Acetone | < 5 mg/dl – | v mg/dl (0.five mmol/l) 40 mg/dl (7 mmol/l) |
| Urobilinogen | < ane mg/dl | 0.4 mg/dl (7 µmol/l) |
| Bilirubin | < 0.2 mg/dl | 0.five mg/dl (9 µmol/l) |
| Blood Erythrocytes Haemoglobin | 0–5 Ery/µl – | five Ery/µl 0.03 mg/dl Hb |
[23]
Medical uses [edit]
Urine examination strips can be used in many areas of the healthcare chain including screening for routine examinations, treatment monitoring, self-monitoring past patients and/or general preventive medicine.
Screening [edit]
Urine test strips are used for screening both in hospitals and in general practice. The aim of screening is early on identification of probable patients by examination of large groups of the population. The importance of screening for diabetes and kidney disease among high-adventure populations is becoming very high.
Treatment monitoring [edit]
Treatment monitoring with the aid of urine test strips allows a health professional to cheque on the results of the prescribed therapy, and if necessary to introduce whatsoever changes into the grade of therapy.
Self-monitoring [edit]
Self-monitoring with urine test strips under the guidance of a health professional is an effective method for monitoring the disease state. This applies particularly to diabetics, where the idea of cocky-monitoring of the metabolic status (determinations of glucose and ketones) is self-evident.
Veterinary [edit]
In veterinary medicine, especially in cats and dogs, the examination strip can be used for urinalysis.
History [edit]
In many cultures urine was once regarded as a mystical fluid, and in some cultures it is nonetheless regarded as such to this day. Its uses accept included wound healing, stimulation of the body's defences, and examinations for diagnosing the presence of diseases.
It was only towards the end of the 18th century that doctors interested in chemistry turned their attention to the scientific ground of urinalysis and to its use in applied medicine.
- 1797 - Carl Friedrich Gärtner (1772–1850) expressed a wish for an easy way of testing urine for disease at the patient's bedside.[24]
- 1797 - William Cumberland Cruikshank (1745–1800) described for the showtime time the property of coagulation on heating, exhibited by many urines.
- 1827 - English language physician Richard Bright describes the clinical symptom of nephritis in "Reports of Medical Cases."
- 1840 - The arrival of chemical urine diagnostics aimed at the detection of pathological urine constituents
- 1850 - Parisian pharmacist Jules Maumené (1818–1898) develops the showtime "test strips" when he impregnated a strip of merino wool with "can protochloride" (stannous chloride). On awarding of a driblet of urine and heating over a candle the strip immediately turned blackness if the urine contained carbohydrate.
- 1883 - English physiologist George Oliver (1841–1915) markets his "Urinary Test Papers"
- approx. 1900 - Reagent papers get commercially obtainable from the chemical company of Helfenberg AG.
- 1904 - A examination for the presence of blood by a moisture-chemical method using benzidine became known.
- approx. 1920 - Viennese chemist Fritz Feigl (1891–1971) publishes his technique of "spot analysis".
- 1930s - Urine diagnostics makes major progress every bit reliability improves and exam performance becomes progressively easier.
- 1950s - Urine examination strips in the sense used today were first fabricated on industrial scale and offered commercially.
- 1964 - The visitor Boehringer Mannheim, today Roche, launched its kickoff Combur test strips.
Even though the test strips take inverse little in appearance since the 1960s, they at present contain a number of innovations. New impregnation techniques, more stable colour indicators, and the steady improvement in colour gradation take all contributed to the fact that the apply of urine test strips has now become established in clinical and full general practice every bit a reliable diagnostic instrument. The parameter menu offered has steadily grown longer in the intervening decades.
Ascorbic acrid interference [edit]
Ascorbic acrid (vitamin C) is known to interfere with the oxidation reaction of the blood and glucose pad on common urine test strips. Some urine examination strips are protected confronting the interference with iodate, which eliminates ascorbic acid by oxidation.[25] Some examination strips include a test for urinary ascorbate.
Urinary sediment [edit]
During routine screening, if a positive exam for leukocytes, blood, protein, nitrite, and a pH greater than 7 is identified, the urine sediment be microscopically analysed to further pinpoint a diagnosis.
Automatic analysers [edit]
Automatic analysis of urine test strips using automated urine test strip analysers is a well-established exercise in mod-solar day urinalysis. They can measure calcium, blood, glucose, bilirubin, urobilinogen, ketones, leukocytes, creatinine, microalbumin, pH, ascorbic acid and protein.[26]
References [edit]
- ^ Yetisen A. M. (2013). "Newspaper-based microfluidic point-of-care diagnostic devices". Lab on a Chip. 13 (12): 2210–2251. doi:10.1039/C3LC50169H. PMID 23652632.
- ^ a b c d due east f Strasinger, Susan M.; Di Lorenzo Schaub, Marjorie (2008). "5". Análisis de orina y de los líquidos corporales (in Spanish) (5ª ed.). Editorial panamericana. pp. 53–76. ISBN978-950-06-1938-7 . Retrieved March 13, 2012.
- ^ http://www.seg-social.es/ism/gsanitaria_es/ilustr_capitulo6/cap6_7_analisorina.htm Archived 2011-03-16 at the Wayback Motorcar language = Spanish
- ^ a b c Strasinger, Susan M.; Di Lorenzo Schaub, Marjorie (2008). "5". Análisis de orina y de los líquidos corporales (in Spanish) (5ª ed.). Editorial panamericana. pp. 56–57. ISBN978-950-06-1938-7 . Retrieved 13 March 2012.
- ^ ADW Diabetes.
- ^ a b c d east f g h i j thou Bayer Multistix reagent strips
- ^ Strasinger, Susan K.; Di Lorenzo Schaub, Marjorie (2008). "4". Análisis de orina y de los líquidos corporales (in Spanish) (5ª ed.). Editorial panamericana. pp. 46–47. ISBN978-950-06-1938-7 . Retrieved 14 March 2012.
- ^ a b c Strasinger, Susan K.; Di Lorenzo Schaub, Marjorie (2008). "five". Análisis de orina y de los líquidos corporales (in Spanish) (5ª ed.). Editorial panamericana. pp. 75–76. ISBN978-950-06-1938-7 . Retrieved 14 March 2012.
- ^ "Urine specific gravity". Medline Plus . Retrieved 30 March 2013.
- ^ Urinalaysis and Torso Fluids Sixth Edition by Susan King Strasinger and Marjorie Schaub Di Lorenzo
- ^ "Your Kidneys and How They Work". National Kidney and Urological Disease Information Clearing Business firm. 2007. Archived from the original on 2011-04-eleven. Retrieved 2009-02-17 .
- ^ Urinalysis and Body Fluids by Susan King Strasinger and Marjorie Schaub Di Lorenzo
- ^ a b c d e Wein, Alan J.; Kavoussi, Louis R.; Novick, Andrew C.; Partin, Alan West.; Peters, Craig A. (2007). "iii". Campbell-Walsh Urología (in Castilian) (9ª ed.). Editorial Médica Panamericana. pp. 97–98. ISBN978-950-06-8268-eight . Retrieved thirteen March 2012.
- ^ Tests for the Identification of Aldehydes and Ketones (in Spanish)
- ^ a b c Wein, Alan J.; Kavoussi, Louis R.; Novick, Andrew C.; Partin, Alan W.; Peters, Craig A. (2007). "3". Campbell-Walsh Urología (in Castilian) (9ª ed.). Editorial Médica Panamericana. p. 104. ISBN978-950-06-8268-8 . Retrieved 13 March 2012.
- ^ a b c Strasinger, Susan Chiliad.; Di Lorenzo Schaub, Marjorie (2008). "5". Análisis de orina y de los líquidos corporales (in Spanish) (5ª ed.). Editorial panamericana. pp. 70–73. ISBN978-950-06-1938-7 . Retrieved 14 March 2012.
- ^ Graff, Laurine (1987). "2". Análisis de orina - Atlas Color (in Spanish) (1ª ed.). Ed. Médica Panamericana. p. 59. ISBN978-950-06-0841-1 . Retrieved fourteen March 2012.
- ^ Graff, Laurine (1987). "two". Análisis de orina - Atlas Colour (in Castilian) (1ª ed.). Ed. Médica Panamericana. p. threescore. ISBN978-950-06-0841-one . Retrieved xiv March 2012.
- ^ a b c Wein, Alan J.; Kavoussi, Louis R.; Novick, Andrew C.; Partin, Alan W.; Peters, Craig A. (2007). "iii". Campbell-Walsh Urología (in Spanish) (9ª ed.). Editorial Médica Panamericana. p. 104. ISBN978-950-06-8268-viii . Retrieved 14 March 2012.
- ^ a b c Strasinger, Susan K.; Di Lorenzo Schaub, Marjorie (2008). "5". Análisis de orina y de los líquidos corporales (in Spanish) (5ª ed.). Editorial panamericana. pp. 73–75. ISBN978-950-06-1938-7 . Retrieved 14 March 2012.
- ^ a b c Strasinger, Susan 1000.; Di Lorenzo Schaub, Marjorie (2008). "v". Análisis de orina y de los líquidos corporales (in Spanish) (5ª ed.). Editorial panamericana. pp. 74–75. ISBN978-950-06-1938-vii . Retrieved 14 March 2012.
- ^ Scheer, KA; Segert, LA; Grammers, GL (1984). "Urine leukocyte esterase and nitrite tests as an help to predict urine culture results". Lab Med. 15 (3): 186–187. doi:10.1093/labmed/15.iii.186.
- ^ (2008) Combur-Exam: Detailed information. Retrieved February 09, 2009, from Roche Diagnostics. Web site: http://world wide web.diavant.com/diavant/CMSFront.html?pgid=3,2,14,1
- ^ Sahnan, Kapil; Blakey, Sarah; Ball, Kathryn; Bagenal, Jessamy; Patel, Biral (January 2013). "I went to the urologist and this is what I brought". Bulletin of the Royal College of Surgeons of England. 95 (1): 43–44. doi:ten.1308/147363513x13500508918656.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Brigden ML, Edgell D, McPherson Thou, Leadbeater A, Hoag Thou (March 1992). "High incidence of significant urinary ascorbic acid concentrations in a west coast population—implications for routine urinalysis". Clin. Chem. 38 (3): 426–31. doi:x.1093/clinchem/38.3.426. PMID 1547565.
- ^ "Archived copy". Archived from the original on 2012-06-30. Retrieved 2013-04-02 .
{{cite web}}: CS1 maint: archived re-create as title (link)
Further reading [edit]
- Compendium Urinalysis: Urinalysis with Test Strips. Dr East F Hohenberger, Dr H Kimling (2002)http://world wide web.diavant.com/diavant/servlet/MDBOutput?fileId=1392
- Strasinger, Susan K.; Di Lorenzo Schaub, Marjorie (2008). "5". Análisis de orina y de los líquidos corporales (in Spanish) (5ª ed.). Editorial panamericana. pp. 56–57. ISBN978-950-06-1938-7 . Retrieved xiv March 2012.
- Graff, Laurine (1987). "2". Análisis de orina - Atlas Colour (in Spanish) (1ª ed.). Ed. Médica Panamericana. p. 60. ISBN978-950-06-0841-i . Retrieved 14 March 2012.
- Wein, Alan J.; Kavoussi, Louis R.; Novick, Andrew C.; Partin, Alan West.; Peters, Craig A. (2007). "3". Campbell-Walsh Urología (in Spanish) (9ª ed.). Editorial Médica Panamericana. p. 104. ISBN978-950-06-8268-8 . Retrieved xiv March 2012.
- Urinalysis Strips Instructions
Source: https://en.wikipedia.org/wiki/Urine_test_strip
0 Response to "what test is used to measure the concentration of urine?"
Post a Comment